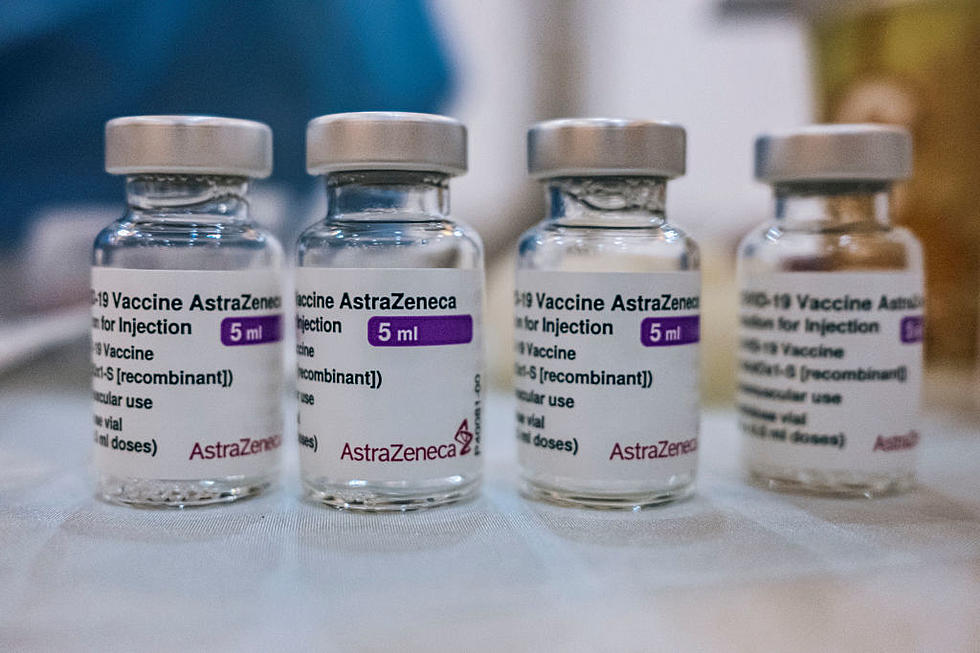
DOH Pushing Anti-COVID Drug That’s Not Yet FDA Approved
The Washington State Department of Health released information Monday about what they say is a new anti-COVID drug targeted toward "high-risk" COVID persons.
Evusheld still awaits FDA approval, it's EUA only now
Evusheld is the 'brand' name for tixagevimab co-packaged with cilgavimab, and the DOH says it's effective in preventing COVID for high-risk persons. It's manufactured and marketed by Astra Zenaca.
DOH said due to extremely short supply it was not readily available in our state, but now apparently thousands of doses are for high-risk persons.
However, according to the FDA, the drug is still under Emergency Use Authorization status, and has not been formally approved. In fact, the FDA say it is an "investigational drug" according to information released by them recently.

It's in the form of an injection, according to the DOH. For more information on this drug, click on the button below.
Goosebumps and other bodily reactions, explained
More From 870 AM KFLD